 |
| इयत्ता आठवी विज्ञान धडा धातू आणि नॉनमेटल्स मराठी स्वाध्याय PDF |
या लेखात, आम्ही विज्ञान धडा धातू आणि नॉनमेटल्स विषयासाठी इयत्ता आठवी मराठी सोल्यूशन्स देऊ. इयत्ता आठवी मधील विद्यार्थी पाठ्यपुस्तकांमध्ये उपस्थित असलेल्या व्यायामांसाठी प्रश्न आणि उत्तरे डाउनलोड आणि कॉपी करण्यास सक्षम असतील.
इयत्ता आठवी विज्ञान धडा धातू आणि नॉनमेटल्साच्या पुस्तकात महाराष्ट्र बोर्डाच्या अभ्यासक्रमातील सर्व प्रश्नांचा समावेश आहे. येथे सर्व प्रश्न पूर्ण स्पष्टीकरणासह सोडवले आहेत आणि डाउनलोड करण्यासाठी विनामूल्य उपलब्ध आहेत. महाराष्ट्र बोर्ड इयत्ता आठवी विज्ञान धडा धातू आणि नॉनमेटल्साचे पुस्तक खाली दिले आहे. आम्हाला आशा आहे की आमच्या इयत्ता आठवी वीच्या विज्ञान धडा धातू आणि नॉनमेटल्साचे पुस्तक तुमच्या अभ्यासात मदत करेल! जर तुम्हाला आमचे इयत्ता आठवी चे पुस्तक आवडले असेल तर कृपया ही पोस्ट शेअर करा.
इयत्ता आठवी विज्ञान धडा धातू आणि नॉनमेटल्स स्वाध्याय
|
मंडळाचे नाव |
Maharashtra Board |
|
ग्रेडचे नाव |
आठवी |
|
विषय |
विज्ञान धडा धातू आणि नॉनमेटल्स |
|
वर्ष |
2022 |
|
स्वरूप |
PDF/DOC |
|
प्रदाता |
|
|
अधिकृत संकेतस्थळ |
mahahsscboard.in |
समाधानासह महाराष्ट्र बोर्ड आठवा स्वाध्याय कसे डाउनलोड करायचे?
महाराष्ट्र बोर्ड आठवी स्वाध्याय PDF डाउनलोड करण्यासाठी खालील स्टेप्स फॉलो करा:
- वेबसाइट- Hsslive ला भेट द्या. 'स्वाध्याय' लिंकवर क्लिक करा.
- महा बोर्ड आठवी स्वाध्याय PDF पहा.
- आता महाराष्ट्र बोर्ड आठवी स्वाध्याय तपासा.
- डाउनलोड करा आणि भविष्यातील संदर्भांसाठी जतन करा.
इयत्ता आठवी विज्ञान धडा धातू आणि नॉनमेटल्स स्वाध्याय उपाय
इयत्ता आठवी स्वाध्याय मधील विद्यार्थी खालील लिंक्सवरून विज्ञान धडा धातू आणि नॉनमेटल्साचे उपाय डाउनलोड करू शकतील.
Class 8 Science Chapter 7 Metals and Nonmetals Textbook Questions and Answers
1. Complete the table:
Question a.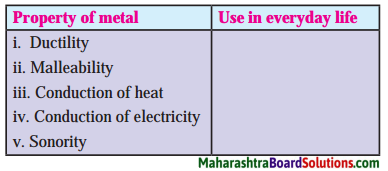
Answer:
| Property of metal | Use in everyday life |
| i. Ductility | i. Gold, silver ornaments |
| ii. Malleability | ii. Aluminium sheets, galvanised sheets |
| iii. Conduction of heat | iii. Stainless steel vessels, copper vessels, boilers |
| iv. Conduction of electricity | iv. Copper wires |
| v. Sonority | v. Brass articles |
2. Identify the odd term.
Question a.
Gold, Silver, Iron, Diamond.
Answer:
Diamond. (Others are metals.)
Question b.
Ductility, Brittleness, Sonority, Malleability.
Answer:
Brittleness. (Other properties are metallic properties.)
Question c.
Carbon, Bromine, Sulphur, Phosphorus.
Answer:
Bromine. (Others are solids.)
Question d.
Brass, Bronze, Iron, Steel.
Answer:
Iron. (Others are alloys.)
3. Give scientific reasons:
Question a.
The stainless steel vessels in kitchen have copper coating on the bottom.
Answer:
- Stainless steel is an alloy of iron ; with carbon, chromium and nickel.
- The conductivity of copper is higher than that of iron in steel. Copper heats uniformly and faster. The time for cooking is reduced, as a result it saves fuel. Hence, the stainless steel vessels in kitchen have copper coating on the bottom.
Question b.
Copper and brass vessels are cleaned with lemon.
Answer:
- Copper undergoes oxidation in air to form black copper oxide. Copper oxide reacts slowly with carbon dioxide in air and gains a green coat. This green substance is copper carbonate.
- Lemon contains acid. The acid dissolves the green coating of basic copper carbonate present on the surface of a tarnished copper and brass vessels and makes them shiny again.
Question c.
Sodium metal is kept in kerosene.
Answer:
- Sodium reacts so vigorously with atmospheric oxygen and water that it catches fire if kept in the open.
- It does not react with kerosene and sinks in it. Hence, to protect sodium and to prevent accidental fires it is always kept in kerosene.
4. Answer the following:
Question a.
What is done to prevent corrosion of metals?
Answer:
By applying a layer of paint, oil, grease or varnish on the surface of a metal to prevent corrosion. Also plating with noncorroding metal is done. Iron is coated with thin layer of zinc. Due to these processes the contact of metal surface with air is lost and corrosion is prevented.
Question b.
What are the metals that make the alloys brass and bronze?
Answer:
The alloy brass is formed from copper and zinc and the alloy bronze is formed from copper and tin.
Question c.
What are the adverse effects of i corrosion?
Answer:
- A reddish coloured deposit (rust) is formed on iron by reaction with oxygen gas.
- A greenish coloured deposit (copper carbonate) is formed on copper by reaction with carbon dioxide.
- A blackish coloured deposit is formed (silver sulphide) on silver.
- Corrosion causes damages to car bodies, bridges, iron railings, ships specially those of iron, silver articles and copper vessels.
Question c.
What are the uses of noble metals?
Answer:
Uses of Noble Metals:
- Gold, silver and platinum are used to prepare ornaments.
- Silver is used in medicines. (It has antibacterial property).
- Gold and silver are also used to make metals and few electronic devices.
- Platinum, palladium metals are used as catalyst.
5. Three experiments to study the process of rusting are given below. Observe the three test tubes and answer the following questions.
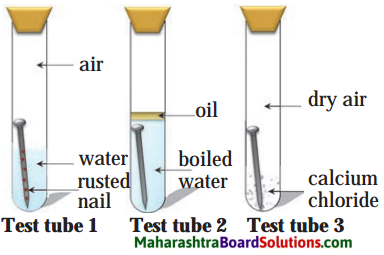
Question a.
Why the nail in the test tube 2 is not rusted?
Answer:
In the test tube 2, oil cuts the supply of air to nail due to which oxidation of nail is prevented and boiled water is free from gases. Hence, the nail in the test tube 2 is not rusted.
Question b.
Why is the nail in the test tube 1 is rusted highly?
Answer:
The nail in the test tube 1 is highly rusted because nail is in contact with water and air. The oxidation process is fast.
Question c.
Would the nail in the test tube 3 get rusted?
Answer:
No change is observed in the test tube 3. Nail remains as it is because the calcium chloride absorbs moisture, making the air dry, thus preventing rusting of the nail.
Project:
Question a.
How is the Varkha or silver foil used in sweets made? Collect the information about which metals are used to make ‘Varkha’.
Class 8 Science Chapter 7 Metals and Nonmetals Additional Important Questions and Answers
Rewrite the sentences after filling the blanks:
Question 1.
Gold of 100% purity is …………… carat gold.
Answer:
Gold of 100% purity is 24 carat gold.
Question 2.
The stainless steel utensils used at home are made of an alloy of iron with carbon, …………… and ……….. .
Answer:
The stainless steel utensils used at home are made of an alloy of iron with carbon, nickel and chromium.
Question 3.
…………….. is used in medicines.
Answer:
Silver is used in medicines.
Question 4.
To prepare ornaments ………… carat gold is used.
Answer:
To prepare ornaments 22 carat gold is used.
Question 5.
Non-metals are ………… conductors of heat and electricity.
Answer:
Non-metals are bad conductors of heat and electricity.
Question 6.
Non-metals form ………….. oxides.
Answer:
Non-metals form acidic oxides.
Question 7.
Some element can be hammered into thin sheets. This property is called …………….. .
Answer:
Some element can be hammered into thin sheets. This property is called malleability.
Question 8.
Iodine and ……………. are the two non – metals having typical metallic lustre.
Answer:
Iodine and diamond are the two non¬metals having typical metallic lustre.
Question 9.
Non-metals form ………. ions by gain of electrons.
Answer:
Non-metals form negative ions by gain of electrons.
Question 10.
During formation of positively charged ion, atom of metal ………….. .
Answer:
During formation of positively charged ion, atom of metal lose electrons.
Rewrite the following statements by selecting the correct options:
Question 1.
Metalloids have properties of …………. .
(a) metals
(b) non-metals
(c) both metals and non-metals
(d) neither metals nor non-metals
Answer:
(c) both metals and non-metals
Question 2.
………….. is a metal.
(a) Hg
(b) S
(c) P
(d) Br
Answer:
(a) Hg
Question 3.
…………… is a non-metal.
(a) Aug
(b) Ag
(c) Br
(d) Cu
Answer:
(c) Br
Question 4.
……………. is a metalloid.
(a) Aluminium
(b) Antimony
(c) Zinc
(d) Mercury
Answer:
(b) Antimony
Question 5.
……………. is a metal which is in liquid form at ordinary temperature.
(a) Gallium
(b) Magnesium
(c) Sodium
(d) Scandium
Answer:
(a) Gallium
Question 6.
…………….. is an acidic oxide.
(a) Na2O
(b) CO2
(c) FeOs
(d) H2O
Answer:
(b) CO2
Question 7.
The valence electron in …………… atom is 1.
(a) magnesium
(b) sodium
(c) silicon
(d) aluminium
Answer:
(b) sodium
Question 8.
The electronic configuration of oxygen is …………….. .
(a) 2, 5
(b) 2, 6
(c) 2, 4
(d) 2, 8, 6
Answer:
(b) 2, 6
Question 9.
Pure ………… is soft.
(a) aluminium
(b) silver
(c) gold
(d) platinum
Answer:
(c) gold
Question 10.
…………… is a highly malleable metal.
(a) Iron
(b) Nickel
(c) Manganese
(d) Aluminium
Answer:
(d) Aluminium
Question 11.
22 carat gold is gold of …………… purity.
(a) 100%
(b) 91.66%
(c) 75%
(d) 44%
Answer:
(b) 91.66%
State whether the following statements are True or False:
Question 1.
Metals are sonorous.
Answer:
True.
Question 2.
Diamond is the softest natural substance.
Answer:
False. (Diamond is the hardest natural substance.)
Question 3.
The density of lithium is lower than water.
Answer:
True.
Question 4.
Sulphur is brown in colour.
Answer:
False. (Sulphur is yellow in colour.)
Question 5.
Germanium is a metalloid.
Answer:
True.
Question 6.
Ornaments are made from 24 carat gold.
Answer:
False. (Ornaments are made from 22 carat gold.)
Question 7.
A reddish coloured deposit is formed on iron by reaction with oxygen.
Answer:
True.
Question 8.
Paladium and rhodium are noble metals.
Answer:
True.
Question 9.
The metal oxides are acidic in nature.
Answer:
False. (The metal oxides are basic in nature.)
Question 10.
The metal reacts with dilute acids to form a metal salt.
Answer:
True.
Identify the odd term:
Question 1.
Magnesium, Mercury, Sodium, Iron.
Answer:
Mercury. (Others metals are in solid state at normal temperature.)
Question 2.
Magnesium, Aluminium, Sulphur, Copper.
Answer:
Sulphur. (Others are metals.)
Question 3.
Iron, Copper, Graphite, Phosphorus.
Answer:
Phosphorus. (Others are good conductors of electricity.)
Question 4.
Tin, Bronze, Steel, Stainless steel.
Answer:
Tin. (Others are alloys.)
Consider the relation between the items in the first pair and write the correlation for second pair:
Question 1.
Mercury : Metal : : Carbon : …………. .
Answer:
Non-metal
Question 2.
Wood : Bad conductor of electricity : : Copper : ………………… .
Answer:
Good conductor or electricity
Question 3.
Gold : Noble metal : : Copper: ……………. .
Answer:
Metal
Question 4.
Iron and carbon : Steel : : Copper and Tin : ……….. .
Answer:
Bronze
Question 5.
0 : 2, 6 : : Mg : …………….. .
Answer:
2, 8, 2.
Match the columns:
Question 1.
| Column ‘A’ | Column ‘B’ |
| 1. Copper | a. Low melting point |
| 2. Sodium | b. Liquid |
| 3. Magnesium | c. High melting point |
| 4. Boron | d. Conduction of heat |
| e. 2, 8, 2 |
Answer:
| Column ‘A’ | Column ‘B’ |
| 1. Copper | d. Conduction of heat |
| 2. Sodium | a. Low melting point |
| 3. Magnesium | e. 2, 8, 2 |
| 4. Boron | c. High melting point |
Question 2.
| Column ‘A’ | Column ‘B’ |
| 1. Silver | a. Copper + tin |
| 2. Mercury | b. Medicines |
| 3. Platinum | c. Thermometer |
| 4. Brass | d. Catalyst |
Answer:
| Column ‘A’ | Column ‘B’ |
| 1. Silver | b. Medicines |
| 2. Mercury | c. Thermometer |
| 3.Platinum | d. Catalyst |
| 4. Brass | a. Copper + tin |
Define the following:
- Ductility: The property due to which a substance can be drawn into a thin wire is called ductility.
- Malleability: The property due to which a substance can be hammered into a thin sheet is called malleability.
- Metalloids: The element which shows the properties of metals as well as those of non-metals is called a metalloid.
- Corrosion: Gases in the air react with metals in presence of moisture to form metal compounds on its surface, this destroys the metal gradually and is called corrosion.
- Alloy: A homogeneous mixture of two or more metals or a homogeneous mixture of metal with non-metals is called alloy.
Answer the following questions in one sentence:
Question 1.
Name an alloy of copper and tin.
Answer:
An alloy of copper and tin is termed as bronze.
Question 2.
Name a metal which is in liquid state at ordinary temperature.
Answer:
Mercury is in liquid state at ordinary temperature.
Question 3.
Name two metals which are malleable.
Answer:
Iron and aluminium are malleable metals.
Question 4.
Name two metals which are ductile.
Answer:
Gold and silver are ductile metals.
Question 5.
Name two metals which are good conductors of heat.
Answer:
Silver and copper are good conductors of heat.
Question 6.
Name two metals which are good conductors of electricity.
Answer:
Copper and aluminium are good conductors of electricity.
Question 7.
Name two non-metals which are in solid state at room temperature.
Answer:
Carbon and sulphur are solids at room temperature.
Question 8.
Name two non-metals which are in gaseous state at room temperature.
Answer:
Hydrogen and oxygen are in gaseous state at room temperature.
Question 9.
Name the non-metal having electrical conductivity.
Answer:
Graphite has electrical conductivity.
Question 10.
Name two non-metals which are lustrous.
Answer:
Iodine and diamond are lustrous in nature.
Question 11.
Name two non-metals having high melting points.
Answer:
Carbon and boron melt at high temperature.
Question 12.
State two metals which can be cut easily with a knife.
Answer:
Sodium and potassium are soft metals and can be cut easily with a knife.
Question 13.
Which is the hardest natural substance?
Answer:
Diamond, which is a form of carbon is the hardest natural substance.
Question 14.
State the property of the metals for which they can be drawn into wires.
Answer:
The property of the metals due to which they can be drawn into wires is called ductility.
Question 15.
State the property of the metals due to which they can be beaten into thin sheets.
Answer:
The property of the metals due to which they can be beaten into thin sheets is called malleability.
Question 16.
Which of the following metals react with cold water? Sodium, iron, copper, potassium.
Answer:
Sodium and potassium metals react with cold water.
Question 17.
Which of the following metals do not react with cold water or hot water?
Answer:
Aluminium and iron do not react with cold water or hot water.
Question 18.
What are the constituents of stainless steel?
Answer:
Iron with carbon, chromium and nickel are the constituents of stainless steel.
Question 19.
State the term used to express the purity of gold.
Answer:
The purity of gold is expressed in carat.
Question 20.
Name two metals having low melting points and boiling points.
Answer:
Sodium and potassium having low melting points and boiling points.
Question 21.
Name a non-metal which is in liquid state at room temperature.
Answer:
Bromine is in liquid state at room temperature.
Question 22.
State the atomic number and electronic configuration of sodium.
Answer:
Atomic number of sodium: 11.
Electronic configuration of sodium (Na): 2, 8, 1.
Question 23.
State the atomic number and electronic configuration of aluminium.
Answer:
Atomic number of aluminium: 13.
Electronic configuration of aluminium (Al): 2, 8, 3.
Question 24.
Give two example of metalloids.
Answer:
Metalloids: Arsenic (As),
germanium (Ge), antimony (Sb).
Question 25.
State the atomic number and electronic configuration of nitrogen.
Answer:
Atomic number of nitrogen: 7.
Electronic configuration of nitrogen (N): 2, 5.
Answer the following questions:
Question 1.
A metal can be drawn into a wire. Explain why.
Answer:
- The property due to which a substance can be draw into a thin wire is called ductility.
- Metals are ductile. Thus, a metal can be draw into a wire.
Question 2.
A metal can be hammered into a i thin sheet. Explain why.
Answer:
- The property due to which a substance can be hammered (or rolled) into a thin sheet without cracking is called malleability.
- Metals are malleable. Thus, a metal can be hammered to form a thin sheet.
Question 3.
State the properties of metals.
Answer:
- Metals have a lustre.
- Metals are malleable. They can be beaten into thin sheets.
- Metals are ductile. They can be drawn into wires.
- They are good conductors of heat and electricity.
- At ordinary temperature, metals are generally solid. (Exception: Mercury is liquid.)
- Metals usually have high density.
Question 4.
State the properties of non-metals.
Answer:
- Non-metals lack lustre.
- As non-metals are brittle, they are not malleable.
- They are not ductile.
- Non-metals are poor conductors of heat and electricity.
- At ordinary temperature, non-metals are in the solid or gaseous state. (Exception: Bromine is liquid.)
- Non-metals have low density in the solid state.
Question 5.
How are metal ions formed? Give two examples.
Answer:
Metals have a tendency to lose their valence electrons to form positively charged ions, called cations.
Examples: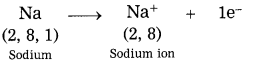
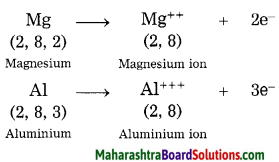
Question 6.
Classify the following elements into metals and non-metals :
Silicon, gold, silver, sulphur, carbon, aluminium, copper and phosphorus.
Answer:
Metals: Gold, silver, aluminium and copper.
Non-metals: Silicon, sulphur, carbon and phosphorus.
Question 7.
How are metal oxides formed? Explain with an example.
Answer:
Metals combine with oxygen in the ’ air to form their oxides.
Metal + Oxygen → Metal oxide
[Note: Reaction/explanation given in the screen are for better understanding of students. The same are not given in the textbook.]
When magnesium burns in air, it combines with oxygen to form magnesium oxide.
2Mg + O2 → 2MgO
Magnesium Oxygen Magnesium oxide
Question 8.
How will you show that metal oxides are basic in nature?
Answer:
1. Take magnesium oxide in a test S tube. Add water in the test-tube. Shake the test-tube. Test the solution with red and blue litmus paper. Blue litmus paper remains as it is while red litmus paper turns blue. This shows that metal oxides are basic in nature.
2. Metal oxides react with an acid to form salt and water. Therefore, metal oxides are basic in nature.
Metal oxide + Acid → + Water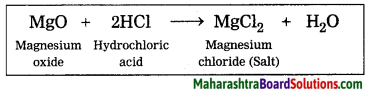
Question 9.
How do metals react with acid? Explain with an example.
Answer:
The metals react with dilute acids to form metal salts. Metals liberate hydrogen gas from dilute acids.
Metal + Dilute acid → Metal salt + Hydrogen gas.
Magnesium reacts with dilute HCl to form magnesium chloride and hydrogen gas.
Question 10.
How do metals react with water? Explain with an example.
Answer:
Most metals do not react with cold water. But some metals like sodium and potassium react with cold water to form their hydroxides and hydrogen gas.
Sodium reacts with water to form sodium hydroxide.
Question 11.
How do non-metals react with oxygen? Explain with an example.
Answer:
Non-metals combine with oxyen to form their oxides.
Non-metal + Oxygen → Non-metal oxide
When carbon burns in air, it combines with oxygen to form carbon dioxide.
Question 12.
How will you show that non- metal oxides are acidic in nature?
Answer:
1. Take non-metal oxide in a test tube. Add water in the test tube. Test the solution with blue and red litmus paper. Red litmus paper remains as it is while blue litmus paper turns red. This shows that non¬metal oxides are acidic in nature.
2. The oxides of non-metals are acidic in nature. They react with bases to form soluble salt and water. Therefore, non-metal oxides are acidic in nature.
C + O2 → CO2
CO2 + 2NaOH → Na2CO3 + H2O
Question 13.
How are non-metal ions formed? Give two examples.
Answer: Non-metals have a tendency to accept electrons in their valence shell to form negatively charged ions called anions.
Examples: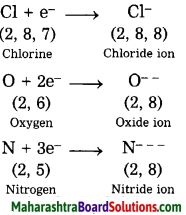
Question 14.
What is meant by noble metals? Give two examples.
Answer:
Some metals like gold, silver, platinum, paladium and rhodium are found in nature in the form of elements. They are not affected by air, water, acids and heat etc. Hence, they are called noble metals.
Give scientific reasons:
Question 1.
Ornaments are generally not made from 24-carat gold.
Answer:
- 24-carat gold is considered as gold of 100% purity.
- 24-carat pure gold is very soft. 100% pure gold breaks due to pressure or gets bent easily. Therefore, ornaments are generally not made from 24-carat gold.
c
Question 2.
Ships are painted at frequent intervals.
Answer:
1. The metal sheets of ships are made from iron.
2. Due to salty seawater, the metal sheets of ships get rusted and corroded. Paints contain metals like zinc or magnesium. This prevents the metal surface of the ship coming into direct contact with salty seawater. Therefore, ships are painted at frequent intervals.
Question 3.
Gold and platinum are called noble elements.
Answer:
- The metals such as gold and platinum are found in nature in the form of elements.
- They are not affected by water, air, acids, heat and also do not take part in chemical reactions. Hence, they are called noble metals.
Explain the following reactions with the help of balanced equations:
Question 1.
Magnesium combines with oxygen.
Answer:
Magnesium combines with oxygen to form magnesium oxide.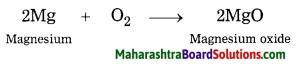
Question 2.
Magnesium oxide reacts with dilute HCl.
Answer:
When magnesium oxide reacts with dilute HCl, magnesium chloride (salt) and water are formed.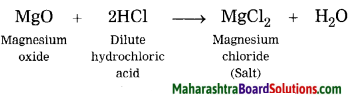
Question 3.
Magnesium reacts with dilute HCl.
Answer:
When magnesium reacts with dil.
hydrochloric acid, magnesium chloride is formed and hydrogen gas is evolved.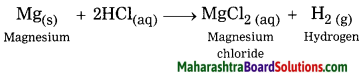
Question 4.
Sodium reacts with water.
Answer:
When sodium reacts with water, sodium hydroxide and hydrogen gas are formed.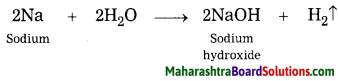
Question 5.
Carbon burns in air.
Answer:
When carbon burns in air, carbon dioxide is formed.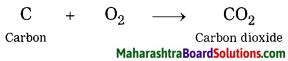
Question 6.
Carbon dioxide reacts with sodium hydroxide.
Answer:
When carbon dioxide reacts with sodium hydroxide, soluble salt sodium ; carbonate and water are formed.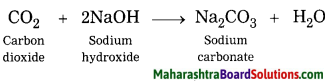
Question 7.
Carbon dioxide reacts with water.
Answer:
When carbon dioxide reacts with water, carbonic acid is formed.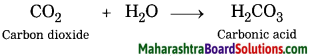
Question 8.
Sulphur dioxide reacts with water.
Answer:
When sulphur dioxide reacts with water, sulphurous acid is formed.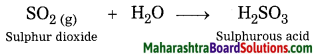
Question 9.
Sulphur trioxide reacts with water.
Answer:
When sulphur trioxide reacts with water, sulphuric acid is formed.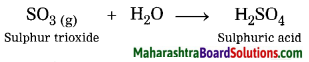
Write short notes:
Question 1.
The noble metals.
Answer:
Some metals like gold, silver, platinum, paladium and rhodium are found in nature in the form of elements. They are not affected by air, water, acids and heat etc. Hence, they are called noble metals.
Uses of Nobel Metals:
- Gold, silver and platinum are used to prepare ornaments.
- Silver is used in medicines. (It has antibacterial property).
- Gold and silver are also used to make metals and few electronic devices.
- Platinum, palladium metals are used as catalyst. .
Question 2.
The purity of gold.
Answer:
1. The purity of gold is measured in carats.
2. Twenty-four carat gold is considered as gold of 100% purity.
3. 24-carat pure gold is very soft. 100% pure gold bends easily or breaks due to pressure. Therefore, copper or silver is added to gold in the necessary proportion (while making ornaments).
4. Generally, 22-carat gold is used in making ornaments.
| Percentage of gold | Carat |
| 100% | 24 |
| 91.66% | 22 |
| 75% | 18 |
| 58.33% | 14 |
| 50% | 12 |
| 41.66% | 10 |
Question 3.
Corrosion.
Answer:
Corrosion: Gases in the air react with metals in presence of moisture to form metal compounds on its surface, this destroys the metal gradually and is called corrosion.
By applying a layer of paint, oil, grease or varnish on the surface of a metal to prevent corrosion. Also plating with noncorroding metal is done. Iron is coated with thin layer of zinc. Due to these processes, the contact of a metal surface with air is lost and corrosion is prevented.
- A reddish coloured deposit (rust) is formed on iron by reaction with oxygen gas.
- A greenish coloured deposit (copper carbonate) is formed on copper by reaction with carbon dioxide.
- A blackish coloured deposit is formed (silver sulphide) on silver.
- Corrosion causes damages to car bodies, bridges, iron railings, ships specially those of iron, silver articles and copper vessels.
Question 4.
Alloys.
Answer:
- A homogeneous mixture of two or more metals or of metals and non-metals is called an alloy.
- They contain metals in specific proportions.
- The physical properties of an alloy are different from those of its constituents, but chemical properties remain the same.
- Copper and tin are used to make an alloy called bronze. It is hard and corrosion resistant.
- When iron and carbon are mixed, an alloy steel is formed. It is a stronger material.
- The alloy, stainless steel is made from iron, carbon, nickel and chromium. It is more durable, clean and does not rust.
Distinguish between the following:
Question 1.
Metals and Non-metals
Answer:
| Metals | Non-metals |
| 1. Metals have lustre. | 1. Non-metals do not have lustre. |
| 2. Metals are malleable. | 2. Non-metals are not malleable. |
| 3. Metals are ductile. | 3. Non-metals are not ductile. |
| 4. Metals are good conductors of heat and electricity. | 4. Non-metals are poor conductors of heat and electricity. |
| 5. At room temperature, metals are in the solid state. (Exception : Mercury is liquid.) | 5. At room temperature, non-metals are in the solid, or gaseous state. (Exception Bromine. It is in liquid state.) |
| 6. Generally, metals have high densities. | 6. Generally, non-metals have lower densities. |
इयत्ता आठवी विज्ञान स्वाध्याय उपाय
- इयत्ता आठवी विज्ञान धडा जिवंत जग आणि सूक्ष्मजीवांचे वर्गीकरण मराठी स्वाध्याय PDF
- इयत्ता आठवी विज्ञान धडा आरोग्य आणि रोग मराठी स्वाध्याय PDF
- इयत्ता आठवी विज्ञान धडा बल आणि दबाव मराठी स्वाध्याय PDF
- इयत्ता आठवी विज्ञान धडा वर्तमान विद्युत आणि चुंबकत्व मराठी स्वाध्याय PDF
- इयत्ता आठवी विज्ञान धडा अणूच्या आत मराठी स्वाध्याय PDF
- इयत्ता आठवी विज्ञान धडा पदार्थाची रचना मराठी स्वाध्याय PDF
- इयत्ता आठवी विज्ञान धडा धातू आणि नॉनमेटल्स मराठी स्वाध्याय PDF
- इयत्ता आठवी विज्ञान धडा प्रदूषण मराठी स्वाध्याय PDF
- इयत्ता आठवी विज्ञान धडा आपत्ती व्यवस्थापन मराठी स्वाध्याय PDF
- इयत्ता आठवी विज्ञान धडा सेल आणि सेल ऑर्गेनेल्स मराठी स्वाध्याय PDF
- इयत्ता आठवी विज्ञान धडा मानवी शरीर आणि अवयव प्रणाली मराठी स्वाध्याय PDF
- इयत्ता आठवी विज्ञान धडा ऍसिड आणि बेसचा परिचय मराठी स्वाध्याय PDF
- इयत्ता आठवी विज्ञान धडा रासायनिक बदल आणि रासायनिक बंध मराठी स्वाध्याय PDF
- इयत्ता आठवी विज्ञान धडा उष्णतेचे मापन आणि परिणाम मराठी स्वाध्याय PDF
- इयत्ता आठवी विज्ञान धडा ध्वनी मराठी स्वाध्याय PDF
- इयत्ता आठवी विज्ञान धडा प्रकाशाचे प्रतिबिंब मराठी स्वाध्याय PDF
- इयत्ता आठवी विज्ञान धडा मानवनिर्मित साहित्य मराठी स्वाध्याय PDF
- इयत्ता आठवी विज्ञान धडा इकोसिस्टम मराठी स्वाध्याय PDF
- इयत्ता आठवी विज्ञान धडा ताऱ्यांचे जीवन चक्र मराठी स्वाध्याय PDF






0 Comments:
Post a Comment